Workshop Day 3 (PAGE 2025)
Workshop Day 3 (PAGE 2025)
OSP-Suite/Event
Modular PBPK Modeling and Quality-Controlled Simulation Workflows in R with OSP Suite V12 (Use Case: Pregnancy PBPK)
Join us for our third Satellite Workshop of the week!
Summary:
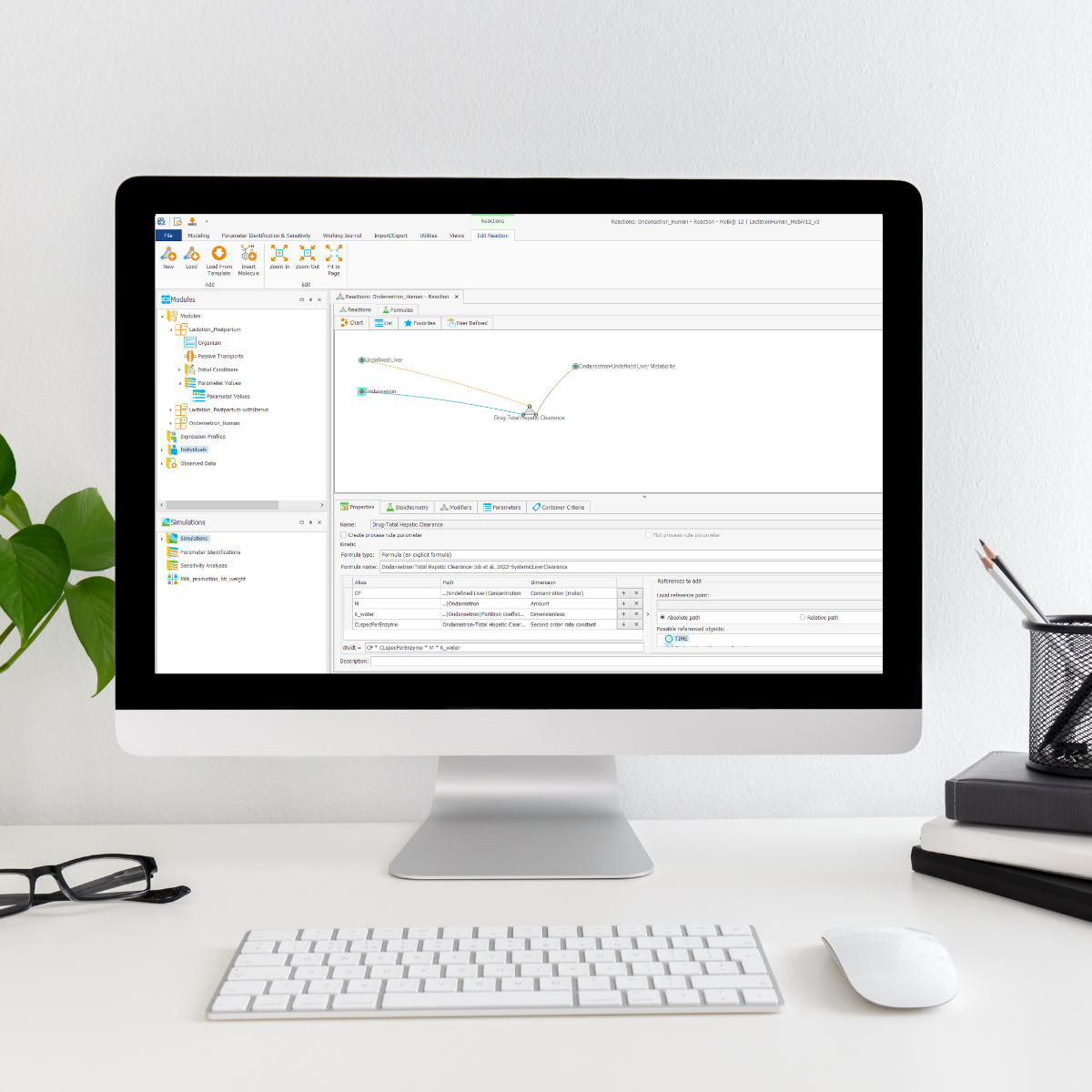
This full-day workshop offers a powerful combination of hands-on modular customized PBPK(-QSP) modeling and quality-controlled simulation workflows in R, leveraging the latest advancements in OSP Suite V12.
🔹 Part 1: Modular PBPK Modeling in OSP Suite V12
Participants will explore the new modularization framework in PK-Sim and MoBi, learning how to extend a PBPK model with a pregnancy module to simulate fetal drug exposure. ESQlabs instructors will guide attendees through:
- Seamlessly adding a pregnancy module to an existing PBPK model.
- Incorporating protein expression data for mechanistic refinements.
- Simulating individual variability using MoBi’s new features.
🔹 Part 2: Quality-Controlled M&S Projects in R with {esqlabsR}
The second half of the workshop focuses on streamlining PBPK-QSP modeling workflows in R using {esqlabsR}, a package designed to enhance reproducibility, transparency, and automation. Participants will:
- Set up and execute simulation scenarios efficiently.
- Generate standardized figures for results visualization.
- Perform sensitivity analyses to assess parameter impact.
- Automate reporting using Markdown templates for regulatory-compliant documentation.
Agenda Highlights:
-
Modularization in OSP Suite V12
-
Pregnancy modeling and fetal exposure simulation
-
{esqlabsR} for structured, reproducible M&S workflows
-
Running and analyzing customized PBPK(-QSP) simulation scenarios
-
Automated sensitivity analysis and visualization
- Generating standardized reports in R
Who Should Attend?
This workshop is ideal for PBPK/QSP modelers, pharmacologists, and regulatory scientists looking to learn about modular customized PBPK(-QSP) modeling in OSP Suite V12 and implement quality-controlled simulation workflows in R. Attendees will leave with practical, hands-on experience in modularization, scenario execution, and automated reporting, essential for regulatory-compliant customized PBPK(-QSP) modeling.
Instructors: Pavel Balazki and Nina Nauwelaerts
The workshop fee includes catering and access to our training materials.
Quantity discount at checkout
Couldn't load pickup availability

Upcoming Events
-
Workshop - Building Scalable PBPK-QSP Models l Boston
Regular price From 300,00 €Regular priceUnit price / per -
Workshop Day 3 (PAGE 2025)
Regular price From 150,00 €Regular priceUnit price / per




